Introduction
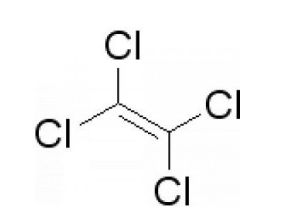
Tetrachloroethylene is an organic compound, also called perchloroethylene, ethylene in all of the hydrogen atoms is substituted by chlorine atoms to generate compound Tetrachloroethylene is an organic compound, also called perchloroethylene, ethylene in all of the hydrogen atoms is substituted by chlorine atoms to generate compound . tetrachloroethylene is relatively stable, it is unlikely to occur in such a manner that the addition reaction of tetrachloroethylene is relatively stable, it is unlikely to occur in such a manner that the addition reaction of .
Michael Faraday in 1821 on the occasion of the first heating and decomposing it, hexachloroethane and perchloroethylene, tetrachloroethylene Michael Faraday in 1821 on the occasion of the first heating and decomposing it, hexachloroethane and perchloroethylene, tetrachloroethylene . chlorine due to the relatively low toxicity, good thermal stability, and has a strong degreasing ability, also can be recovered and recycled, and hence is widely used in the dry - cleaning industry, from 1940 to 2012, the dry - cleaning process has been safe and successful at over 60 years,the clothes to be recognized as a good dry cleaning solvent chlorine due to the relatively low toxicity, good thermal stability, and has a strong degreasing ability, also can be recovered and recycled, and hence is widely used in the dry - cleaning industry, from 1940 to 2012, the dry - cleaning process has been safe and successful at over 60 years,the clothes to be recognized as a good dry cleaning solvent .
With improved quality of life, human impact on the environment, on the side - effects of a variety of daily necessities are also more and more attention, therefore, on the toxicity of tetrachloroethylene were also concerned about With improved quality of life, human impact on the environment, on the side - effects of a variety of daily necessities are also more and more attention, therefore, on the toxicity of tetrachloroethylene were also concerned about . 3 3.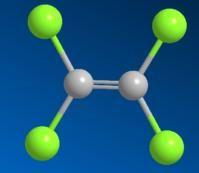
Physicochemical Characteristics
Physical Properties
Nose: The smell of ether Nose: The smell of ether . having similar solubility: it can dissolve a variety of substances (such as rubber, resin, fat, aluminum trichloride, sulfur, iodine, mercuric chloride) having similar solubility: it can dissolve a variety of substances (such as rubber, resin, fat, aluminum trichloride, sulfur, iodine, mercuric chloride) .
with ethanol, ether, chloroform, benzene miscible with ethanol, ether, chloroform, benzene miscible . vapor density (air = 1): 5. The vapor pressure 83: 13 mm Hg (20 C) having a refractive index n20 / D 1. 505 (lit.) Storage: 6 - 0. degree. C. freezing point: - 22. 0. degree. C. Stability: stable saturation vapor pressure (kPa): 2 - 11 (20. degree. C.) heat of combustion (kJ / mol): 679. 3 critical temperature (. degree. C.):347. The critical pressure (MPa) 1: 9. 74 of octanol / water partition coefficient: 2. 88
Chemical Properties
Forbidding substances: alkali, the active metal powder, alkali metal Forbidding substances: alkali, the active metal powder, alkali metal . generally does not burn, but prolonged exposure to open flames and being stable at high temperatures by burning generally does not burn, but prolonged exposure to open flames and being stable at high temperatures by burning .
generated by thermal decomposition of high toxic and corrosive fume generated by thermal decomposition of high toxic and corrosive fume . 1 with chloroform to an addition reaction;with the carbene addition to produce a 6 - chloro - cyclopropane 1 with chloroform to an addition reaction;with the carbene addition to produce a 6 - chloro - cyclopropane .
7 7.
Use
1, as the organic solvent, the dry fire - extinguishing agents, and the extraction agent, the given smoke agent, desulfurizing agent and the like;2, used as anthelmintic 1, as the organic solvent, the dry fire - extinguishing agents, and the extraction agent, the given smoke agent, desulfurizing agent and the like;2, used as anthelmintic . to hookworm which possess paralysis action, however, tetrachloroethylene can be administered orally after absorption from the digestive tract, there are some side effects, mainly in the central nervous system, and can cause liver and kidney damage so to hookworm which possess paralysis action, however, tetrachloroethylene can be administered orally after absorption from the digestive tract, there are some side effects, mainly in the central nervous system, and can cause liver and kidney damage so .
, are typically used as veterinary medicine;3, may be used to produce a metal detergent, a liquid for cleaning car or motorcycle main raw materials;they could be used for cleaning the circuit board;4, was used as fabric finishing agent , are typically used as veterinary medicine;3, may be used to produce a metal detergent, a liquid for cleaning car or motorcycle main raw materials;they could be used for cleaning the circuit board;4, was used as fabric finishing agent . tetrachloroethylene a.k. a., a degreaser, leather products, the manufacturer of the hide tanning, fur degreasing cleaning;5, can also be used for the synthesis of the fluorine - containing organic compounds such as trichloroethylene and
1 1.
Preparation Method
Ethylene Method
may be co - production of trichloroethylene and perchloroethylene, divided the following two method may be co - production of trichloroethylene and perchloroethylene, divided the following two method . 1, direct chlorination of ethylene and chlorine in the presence of a catalyst containing FeCl3 of 1,2 - dichloroethane solution of 280 to 450 DEG C for reaction to produce 1,2 - dichloroethane, trichloroethylene and tetrachloroethylene chlorination to, after distillation, respectively, with NH3, neutralizing, washing, drying, and drying the 1, direct chlorination of ethylene and chlorine in the presence of a catalyst containing FeCl3 of 1,2 - dichloroethane solution of 280 to 450 DEG C for reaction to produce 1,2 - dichloroethane, trichloroethylene and tetrachloroethylene chlorination to, after distillation, respectively, with NH3, neutralizing, washing, drying, and drying the .
2, oxychlorination method: chlorine and ethylene to produce 1,2 - dichloroethane, 1,2 - dichloroethane and chlorine, oxygen and CuCl 2 in KCl as catalyst and 425 c,138 - 207 kPa is carried out under the condition that the oxychlorination reaction, cooling the product, washing, drying, distillation, high - purity product 2, oxychlorination method: chlorine and ethylene to produce 1,2 - dichloroethane, 1,2 - dichloroethane and chlorine, oxygen and CuCl 2 in KCl as catalyst and 425 c,138 - 207 kPa is carried out under the condition that the oxychlorination reaction, cooling the product, washing, drying, distillation, high - purity product .
Hydrocarbon Oxidation
containing methane, ethane, propane, propylene and the like of the hydrocarbon mixture is 50 - 500. degree. C. for the pyrolysis of chlorinated, chlorinated hydrocarbons of the mixture, and after rectification and separated into different product
Acetylene Method
acetylene chloride and heating the chlorination of 1, 1, 2, 2 - tetrachloroethane, trichloroethylene have alkali dehydrochlorination, chlorination of pentachloroethane, followed by alkaline dehydrochlorination of tetrachloroethylene was acetylene chloride and heating the chlorination of 1, 1, 2, 2 - tetrachloroethane, trichloroethylene have alkali dehydrochlorination, chlorination of pentachloroethane, followed by alkaline dehydrochlorination of tetrachloroethylene was . due to acetylene, ethylene or the like has been gradually replaced by due to acetylene, ethylene or the like has been gradually replaced by .
1 1.
Hazard
Toxicity: moderate class Toxicity: moderate class .
Health Hazard
Pleconaril has stimulation and esthetic action Pleconaril has stimulation and esthetic action . inhalation poisoning with acute upper respiratory tract irritation, lacrimation, salivation, inhalation poisoning with acute upper respiratory tract irritation, lacrimation, salivation, .
accompanied by dizziness, headache, nausea, ataxia - like symptoms and drunken accompanied by dizziness, headache, nausea, ataxia - like symptoms and drunken . orally after the dizziness, headache, drowsiness, nausea, vomiting, abdominal pain, blurred vision, numbness of limbs, even excited, convulsions and even coma, orally after the dizziness, headache, drowsiness, nausea, vomiting, abdominal pain, blurred vision, numbness of limbs, even excited, convulsions and even coma, .
lethal chronic effects: fatigue, dizziness, nausea, drunkenness and lethal chronic effects: fatigue, dizziness, nausea, drunkenness and . can have liver damage can have liver damage .
repeated skin contact may cause dermatitis and eczema repeated skin contact may cause dermatitis and eczema ., research has shown women during pregnancy if too much contact of tetrachloroethylene,newborns will suffer from cleft lip and neurological birth defects risk , research has shown women during pregnancy if too much contact of tetrachloroethylene,newborns will suffer from cleft lip and neurological birth defects risk .
6 2 6 2.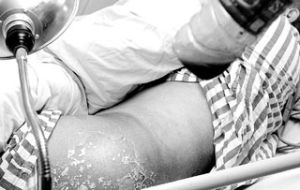
Environmental Hazards
released into the surrounding atmosphere in the majority of tetrachloroethylene, due to the action of sunlight, to form hydrogen chloride, trichloroacetic acid and carbon dioxide as a product of tetrachloroethylene in water released into the surrounding atmosphere in the majority of tetrachloroethylene, due to the action of sunlight, to form hydrogen chloride, trichloroacetic acid and carbon dioxide as a product of tetrachloroethylene in water . rapidly evaporate the water almost do not degrade the rapidly evaporate the water almost do not degrade the .
compounds in groundwater is stable, which is made due to industrial spillage and waste accumulation caused groundwater pollution increasing the incidence of such considerations compounds in groundwater is stable, which is made due to industrial spillage and waste accumulation caused groundwater pollution increasing the incidence of such considerations .
Hazardous Characteristics
generally does not burn, but prolonged exposure to open flames and being stable at high temperatures by burning generally does not burn, but prolonged exposure to open flames and being stable at high temperatures by burning . generated by thermal decomposition of high toxic and corrosive gas with the active metal powder (such as magnesium, aluminum, etc.) can react, causing the
decomposition when exposed to high temperature may be due to induced decomposition, can cause vessel burst and explosion accident or decomposition when exposed to high temperature may be due to induced decomposition, can cause vessel burst and explosion accident or . combustion (decomposition) product: hydrogen chloride, phosgene combustion (decomposition) product: hydrogen chloride, phosgene .
Environmental Standards
The Former Soviet Union
Workplace air - determination of harmful substances in the maximum allowable concentration of 10mg / m3 in the former Soviet Union (1978) in ambient air, to the maximum allowable concentration 0. 06mg / m3 (annual averages)
China
China (GHZB1 - 1999) Surface water environment quality standard (Class I, II, III) waters and 0.005mg / L) of China (GB8978 - 1996 Comprehensive Sewage Discharge Standard level: 0. 1mg / L II: 0. 2mg / L Level 3: 0. 5mg / L
Japan
water: 0. 01mg / L wastewater: 0. 1mg / L, the extraction of soil: 0. 01mg / L olfactory threshold concentration: 50 ppm 1
Treatment Measures
First Aid Measures
Skin contact: Remove contaminated clothes, rinse the skin thoroughly by soap water and fresh water Skin contact: Remove contaminated clothes, rinse the skin thoroughly by soap water and fresh water . Eye contact: Uplift the eyelids, rinse them with flowing fresh water or physiological saline hospitalizing Eye contact: Uplift the eyelids, rinse them with flowing fresh water or physiological saline hospitalizing .
. Inhalation: remove to fresh air quikly Inhalation: remove to fresh air quikly .
. keep respiratory tract unobstructed.Such as difficulty breathing, To oxygen.Such as
breathing stops, conduct artificial respiration immediately hospitalized breathing stops, conduct artificial respiration immediately hospitalized . .
Ingestion: Drink as ample water, induce vomiting Ingestion: Drink as ample water, induce vomiting . .
doctor doctor.
FIRE - FIGHTING MEASURES
fire - extinguishing method: Firefighters should wear breathing apparatus fire - extinguishing method: Firefighters should wear breathing apparatus . spraying water to cool down vessels in fire scene till the fire extinction is spraying water to cool down vessels in fire scene till the fire extinction is .
Extinguishing agents: fog water, foam, dry chemical, carbon dioxide, and sand Extinguishing agents: fog water, foam, dry chemical, carbon dioxide, and sand .
Emergency Leakage Treatment
Rapidly evacuate workers of contaminated area to safety zone, conduct isolation, and strictly limit access to or Rapidly evacuate workers of contaminated area to safety zone, conduct isolation, and strictly limit access to or . It's suggested that emergency treatment staff wear positive pressure self contained breathing apparatus and wear protective clothing to enter the site from the windward of It's suggested that emergency treatment staff wear positive pressure self contained breathing apparatus and wear protective clothing to enter the site from the windward of .
. .
wherever possible to cut off the leakage source and prevent the flow into the sewer drainage such limited space as wherever possible to cut off the leakage source and prevent the flow into the sewer drainage such limited space as . small absorb with sand or other non - combustible materials, it is also possible to use the small absorb with sand or other non - combustible materials, it is also possible to use the .
incombustible emulsion dispersant made of brushing, washing after dilution into the wastewater system incombustible emulsion dispersant made of brushing, washing after dilution into the wastewater system . large number of leak: to build a causeway or trenching asylum large number of leak: to build a causeway or trenching asylum .
Cover with foam to mitigate vapor hazard Cover with foam to mitigate vapor hazard . by means of a pump into a tank truck or special collector, reclaim it or transport to waste fields for disposal by means of a pump into a tank truck or special collector, reclaim it or transport to waste fields for disposal .
Handling And Storage
Precautions for operation: closed operation, strengthen the ventilation Precautions for operation: closed operation, strengthen the ventilation . operation personnel must receive special trainings and strictly followed operation personnel must receive special trainings and strictly followed .
Recommend wearing self - contained type gas masks (half mask), safety chemical goggles, wear the ventilated chemical protective suits, protective gloves Recommend wearing self - contained type gas masks (half mask), safety chemical goggles, wear the ventilated chemical protective suits, protective gloves . away from the tinder and heat sources, workplace smoking away from the tinder and heat sources, workplace smoking .
Use explosion - proof device of ventilation system and Use explosion - proof device of ventilation system and . prevents vapor from escaping to the air of workplace in prevents vapor from escaping to the air of workplace in .
avoided with alkalis, and active metal powders, alkali metal contact avoided with alkalis, and active metal powders, alkali metal contact . Handle with care to,to prevent damage of packages and containers shall be equipped with corresponding Handle with care to,to prevent damage of packages and containers shall be equipped with corresponding .
varieties and quantities of fire equipment and emergency treatment facilities varieties and quantities of fire equipment and emergency treatment facilities . emptying of the container may still contain hazardous substances emptying of the container may still contain hazardous substances .
2 2.
Discard
. disposal by incineration is mixed with fuel then burned disposal by incineration is mixed with fuel then burned .
incinerator of hydrogen halide by acid scrubber to remove incinerator of hydrogen halide by acid scrubber to remove . 2 2.
The United States Has Used For The Dry Cleaning Industry
California's dry - cleaning industry to 2023 before January 1, ending the use of tetrachloroethylene, and the EPA approved California's dry - cleaning industry to 2023 before January 1, ending the use of tetrachloroethylene, and the EPA approved . 5 5.