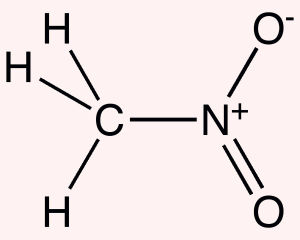
Physical Properties
relative vapor density (air = 1): 2. 11 saturated vapor pressure (kPa): 3.71 (20. degree. C.) heat of combustion (kJ / mol): 708. 1 (c) critical temperature: 315 and a critical pressure (MPa): 6. 30 flash point (C): 35 upper explosion limit% (V / V): 63.0 ignition temperature (. degree. C.): 415 of the lower explosion limit% (V / V): 7. 1 Solubility: soluble in water, alcohol as a colorless oil liquid with alcohol, ether, carbon tetrachloride, dimethyl formamide and the like and a water immiscible organic solvent capable of dissolving the dye with alcohol, ether, carbon tetrachloride, dimethyl formamide and the like and a water immiscible organic solvent capable of dissolving the dye .
, grease, wax, cellulose derivatives, such as a resin, especially a cellulose nitrate,Cellulose acetate has good dissolving capacity , grease, wax, cellulose derivatives, such as a resin, especially a cellulose nitrate,Cellulose acetate has good dissolving capacity . capable of dissolving the aromatic, but not with the paraffins, naphthenes, mixing capable of dissolving the aromatic, but not with the paraffins, naphthenes, mixing .
such a selection feature can be used for the separation of hydrocarbon, lubricating oil refining such a selection feature can be used for the separation of hydrocarbon, lubricating oil refining .
Chemical Properties
1, nitromethane and all of nitroalkanes are easily dissolved in the presence of anhydrous aluminum chloride, which can be used for preparing the content of about 50% of the solution after dissolution of the 1, nitromethane and all of nitroalkanes are easily dissolved in the presence of anhydrous aluminum chloride, which can be used for preparing the content of about 50% of the solution after dissolution of the . formed products of the addition of AlCl3 - RNO2 for hydrocarbons of the alkylation reaction, the catalytic role of the AlCl3 than strong formed products of the addition of AlCl3 - RNO2 for hydrocarbons of the alkylation reaction, the catalytic role of the AlCl3 than strong .
. the acidity of the solution of the product which is inflammable, explosive, the acidity of the solution of the product which is inflammable, explosive, .
the the . wore protective gear is non - hygroscopic, violent impact wore protective gear is non - hygroscopic, violent impact .
2 there is a risk of explosion, the litmus test of nitromethane solution was acidic, i.e., 0. 01 mol / L aqueous solution of pH 6. 12;a saturated aqueous solution of pH 4. 01;water saturated nitro methane (ch3n02) was pH 4. 82 nitro methane have tautomeric, containing traces of acid type structure, in the water, the tautomeric constant KT = 1.1 * 10 - 17
acid type oxygen atoms in the at least one hydrogen atom is quite lively, smooth generation of protons, which is acid, can form salts with the alkali on acid type oxygen atoms in the at least one hydrogen atom is quite lively, smooth generation of protons, which is acid, can form salts with the alkali on . nitromethane with sodium hydroxide to form sodium salt of an explosive, this sodium salt with aldehyde to nucleophilic addition, generate a beta nitro alcohol, for example with formaldehyde in an alkaline solution in the reaction of beta - nitro ethanol nitromethane with sodium hydroxide to form sodium salt of an explosive, this sodium salt with aldehyde to nucleophilic addition, generate a beta nitro alcohol, for example with formaldehyde in an alkaline solution in the reaction of beta - nitro ethanol .
beta - nitro alcohols to dehydration becomes unsaturated nitro compound,For example nitro methane and benzene formaldehyde generated omega - nitrostyrene beta - nitro alcohols to dehydration becomes unsaturated nitro compound,For example nitro methane and benzene formaldehyde generated omega - nitrostyrene . Furthermore, nitro methane may be reduced to methylamine Furthermore, nitro methane may be reduced to methylamine .
3. Stability: stable 4, Incompatibility: strong reductants, acids, alkalis, halogenated alkanes, metal hydrides, metal alkyl oxides, ammonia, amines, and the like 4, Incompatibility: strong reductants, acids, alkalis, halogenated alkanes, metal hydrides, metal alkyl oxides, ammonia, amines, and the like .
5, to avoid contact of the conditions: shaking, heated 5, to avoid contact of the conditions: shaking, heated . 6 Polymerization hazard: no polymeric 6 Polymerization hazard: no polymeric .
7, decomposition products: nitrogen 8, is inflammable and its vapour can form an explosive mixture with air 7, decomposition products: nitrogen 8, is inflammable and its vapour can form an explosive mixture with air . shaken and heated or inorganic bases, oxidizing agents, hydrocarbons, amines, aluminium trichloride, hexamethyl benzene can cause combustion explosion shaken and heated or inorganic bases, oxidizing agents, hydrocarbons, amines, aluminium trichloride, hexamethyl benzene can cause combustion explosion .
combustion decomposition,Non - inflammable, emits poisonous gas combustion decomposition,Non - inflammable, emits poisonous gas .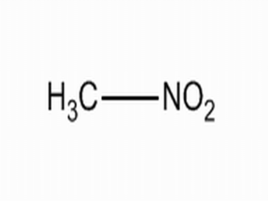
Preparation
1, methane vapor phase nitration method: sprayed in dilute nitric acid so as to be gasified, with preheated gas of methane (natural gas), nitric acid, methane, water vapor remains at a certain proportion of the mixture in the molten salt to 1, methane vapor phase nitration method: sprayed in dilute nitric acid so as to be gasified, with preheated gas of methane (natural gas), nitric acid, methane, water vapor remains at a certain proportion of the mixture in the molten salt to . as heating medium conduits in the reactor, at atmospheric pressure and 450 - 550 DEG C under the condition of direct nitration as heating medium conduits in the reactor, at atmospheric pressure and 450 - 550 DEG C under the condition of direct nitration .
reaction product to condensation and water absorption, of nitro - methane was obtained by distilling an aqueous solution of the crude nitro methane, washing, distillation and finished product consumption reaction product to condensation and water absorption, of nitro - methane was obtained by distilling an aqueous solution of the crude nitro methane, washing, distillation and finished product consumption . ton industrial grade nitric acid 5500kg, natural gas (CH4) is larger than or equal to 95% of a 20000 (2) under the standard condition ton industrial grade nitric acid 5500kg, natural gas (CH4) is larger than or equal to 95% of a 20000 (2) under the standard condition .
,dimethyl sulfate by reaction with sodium nitrite: nitrite and dimethyl sulfate were added to the reaction in a reactor, the reaction product was subjected to condensation, distillation, cooling the layered product ,dimethyl sulfate by reaction with sodium nitrite: nitrite and dimethyl sulfate were added to the reaction in a reactor, the reaction product was subjected to condensation, distillation, cooling the layered product . Furthermore, it is also possible to adopt sodium nitrite reacted with sodium chloroacetate, and then the produced nitrite Furthermore, it is also possible to adopt sodium nitrite reacted with sodium chloroacetate, and then the produced nitrite .
and and . haloalkane reaction are also commercially available other lower alkanes (ethane, propane) chlorine phase nitration directly, but the reaction product is a nitro methane, nitro ethane, nitro propane mixture haloalkane reaction are also commercially available other lower alkanes (ethane, propane) chlorine phase nitration directly, but the reaction product is a nitro methane, nitro ethane, nitro propane mixture .
Application
1. In this way, the adhesive has been used predominantly as a polar solvent is used, may be miscible with many organic compounds can dissolve a cellulose derivative, a resin, a dye, a lubricant such as grease, etc., especially on nitro - cellulose, cellulose acetate, polyacrylonitrile, polyester, wax, etc. have a good solubility to 2, as the aerosol propellant, rocket fuel and manufacturing explosives, dyes etc.
3, used as a solvent, and rocket fuel, gasoline additives and in organic synthesis and 3, used as a solvent, and rocket fuel, gasoline additives and in organic synthesis and . 4, nitro methane has large polar, miscible with many organic compounds, is a good solvent, may be used as cellulose nitrate, cellulose acetate, a vinyl resin,Polyacrylate pigment and beeswax etc. of solvent
also are useful for the preparation of explosives, rocket, fuel, medicines, dyes, pesticides, fungicides, stabilizers, surface active agent and additive etc.
Precautions
Nitromethane nitro methane (control information) are easy to explode the goods in accordance with the Regulations on the Safety Administration of Hazardous Chemicals Control Nitromethane nitro methane (control information) are easy to explode the goods in accordance with the Regulations on the Safety Administration of Hazardous Chemicals Control . Hazard overview by the department of public health hazard: It is mainly caused the central nervous system damage, liver and kidney damage can also cause methemoglobinemia Hazard overview by the department of public health hazard: It is mainly caused the central nervous system damage, liver and kidney damage can also cause methemoglobinemia .
. Acute poisoning: Quick inhalation of high concentrations of vapour occur in CGF is giddy, limply, dyspnea, cyanosis, unconsciousness, seizure Acute poisoning: Quick inhalation of high concentrations of vapour occur in CGF is giddy, limply, dyspnea, cyanosis, unconsciousness, seizure .
on on . with a mild stimulation respiratory tract mucous membrane can occur liver, kidney damage, secondary to nephrotic with a mild stimulation respiratory tract mucous membrane can occur liver, kidney damage, secondary to nephrotic .
met - hemoglobin content in blood increased met - hemoglobin content in blood increased . Explosive hazard: the goods Inflammable, with irritability Explosive hazard: the goods Inflammable, with irritability .
Emergency measures for skin contact:Remove the contaminated clothes, rinse the skin thoroughly by soap water and fresh water Emergency measures for skin contact:Remove the contaminated clothes, rinse the skin thoroughly by soap water and fresh water . Eye contact: Uplift the eyelids, rinse them with flowing fresh water or physiological saline hospitalizing Eye contact: Uplift the eyelids, rinse them with flowing fresh water or physiological saline hospitalizing .
. Inhalation: remove to fresh air quikly Inhalation: remove to fresh air quikly .
. keep respiratory tract unobstructed.Such as difficulty breathing, To oxygen.Such as
breathing stops, conduct artificial respiration immediately hospitalized breathing stops, conduct artificial respiration immediately hospitalized . .
Ingestion: Drink as ample water, induce vomiting hospitalizing Ingestion: Drink as ample water, induce vomiting hospitalizing . .
firefighting measures hazardous combustion products: carbon monoxide, carbon dioxide, nitrogen oxide firefighting measures hazardous combustion products: carbon monoxide, carbon dioxide, nitrogen oxide . fire - extinguishing method: Remove the vessels to open area if possible fire - extinguishing method: Remove the vessels to open area if possible .
spraying water to cool down vessels in fire scene till the fire extinction is spraying water to cool down vessels in fire scene till the fire extinction is . Extinguishing agents: fog water, foam, carbon dioxide, dry powder, Extinguishing agents: fog water, foam, carbon dioxide, dry powder,.
sandy Emergency leak treatment: Rapidly evacuate workers of contaminated area to safety zone, conduct isolation, and strictly limit access to or cut off fire sandy Emergency leak treatment: Rapidly evacuate workers of contaminated area to safety zone, conduct isolation, and strictly limit access to or cut off fire . .
It's suggested that emergency treatment staff wear positive - pressure inhaler and gas - protection suit It's suggested that emergency treatment staff wear positive - pressure inhaler and gas - protection suit . .
wherever possible to cut off the leakage source and prevent the flow into the sewer drainage such limited space as wherever possible to cut off the leakage source and prevent the flow into the sewer drainage such limited space as . small with sand, vermiculite or other inert materials to abosorb small with sand, vermiculite or other inert materials to abosorb .
Large spills: Cover with foam to mitigate vapor hazard to build a causeway or trenching asylum Large spills: Cover with foam to mitigate vapor hazard to build a causeway or trenching asylum . .
spray water cooling and dilution steam, to protect the field, the leakage of the composition is diluted such that the incombustible spray water cooling and dilution steam, to protect the field, the leakage of the composition is diluted such that the incombustible . for explosion proof pump into a tank truck or special collector,for recovery or delivering to a waste disposal plant for disposal for explosion proof pump into a tank truck or special collector,for recovery or delivering to a waste disposal plant for disposal .
Handling and Storage Precautions for operation: closed operation, full exhaust Handling and Storage Precautions for operation: closed operation, full exhaust . operation personnel must receive special trainings and strictly followed operation personnel must receive special trainings and strictly followed .
It is recommended that the operator wear filter type gas masks (half mask), safety chemical goggles, companion b. a biohazard suit, rubber gloves oil away from the tinder and heat sources, workplace smoking oil away from the tinder and heat sources, workplace smoking .
of the ventilation system and explosion proof type equipment of the ventilation system and explosion proof type equipment . prevents vapor from escaping to the air of workplace in prevents vapor from escaping to the air of workplace in .
avoid oxidant, reductant, Acids, Alkalis contact avoid oxidant, reductant, Acids, Alkalis contact . Handle with care to,to prevent damage of packages and containers shall be equipped with corresponding Handle with care to,to prevent damage of packages and containers shall be equipped with corresponding .
varieties and quantities of fire equipment and emergency treatment facilities varieties and quantities of fire equipment and emergency treatment facilities . emptying of the container may still contain hazardous substances emptying of the container may still contain hazardous substances .
storage: Store in a cool, well - ventilated away from the fire and storage: Store in a cool, well - ventilated away from the fire and ., , .
. the heat - source - holding container should be sealed with oxidizing agents, reducing agents, acids, alkalis etc. shall be stored separately, and avoid mixed storage adopt explosion - proof lighting
, , . ventilation facilities easy to generate sparkle shall be prohibited to use machinery and tools shall be prepared in storage area ventilation facilities easy to generate sparkle shall be prohibited to use machinery and tools shall be prepared in storage area .
emergency treatment facilities and suitable housing materials emergency treatment facilities and suitable housing materials .